Understanding HPV (Human Papillomavirus): A Comprehensive Guide to Prevention, Risks, and Vaccination
Human Papillomavirus (HPV) stands as one of the most widespread and multifaceted groups of viruses affecting millions globally. Its implications...
2 min read
Shehnaz Sheikh : Oct 8, 2023 10:43:08 PM

"Corporations have neither bodies to be punished, nor souls to be condemned; they therefore do as they please" - Edward Thurlow, 1st Baron Thurlow (During the prosecution of the East India Company, 1874)
Ina world where businesses exert considerable power over various aspects of society, pharmaceutical giants stand tall with their vast reach and undeniable influencer.
While it's not uncommon for these behemoths to find themselves in legal crosshairs, the Johnson & Johnson narrative stands apart. This titan, once synonymous with trust and household safety, now faces allegations that could tarnish its legacy.
The core of this controversy revolves around the potential endangerment of countless lives due to purported exposure to carcinogenic substances.
Through this article, we seek to explore the transformation of Johnson & Johnson from a beacon of trust to a subject of widespread scrutiny.
One of the most alarming claims against Johnson & Johnson is the purported presence of asbestos, a known carcinogenic agent, in its iconic baby powder.
The severity of this accusation is underscored by the potential health hazards linked with asbestos exposure, which range from respiratory issues to life-threatening cancers.
The alarm bells rang louder in 2019 when the company opted for a recall of 33,000 baby powder bottles in the U.S. This decision followed the detection of asbestos traces by health regulators.
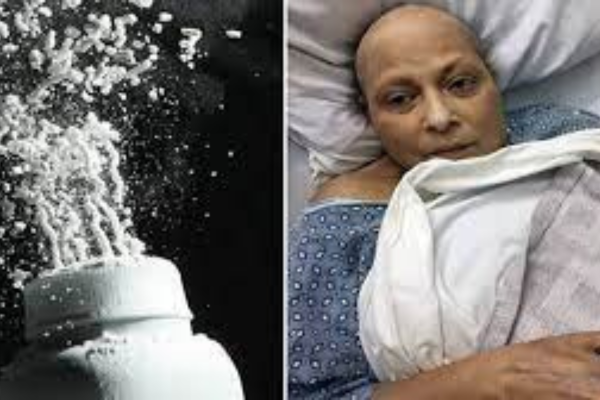
In the face of mounting concerns, Johnson & Johnson has been vocal in its defencse. The company has continuously emphasized that the recall was a proactive measure, taken out of an abundance of caution for the public's well-being, rather than an acknowledgement of any inherent product flaw.
While the corporation remains steadfast in its claims, investigative endeavors by esteemed publications like The New York Times and Reuters in 2018 paint a more convoluted picture.
According to their findings, Johnson & Johnson might have been aware of the asbestos concerns plaguing its talc products for decades, casting shadows over the brand's transparency and commitment to consumer safety.
By the dawn of 2020, the repercussions of the controversy were tangible evident in the company's financial disclosures. Johnson & Johnson reported to the Securities and Exchange Commission (SEC) that it had earmarked a staggering $3.9 billion for potential litigation expenses.
With lawsuits exceeding 25,000, one can't help but ponder the magnitude of the issue. Yet, despite the financial implications and the weight of public sentiment, the conglomerate continues to refute any allegations of misconduct.

Echoing the global trepidations, the FDA in Maharashtra, a significant state in India, has curtailed Johnson & Johnson's operations, revoking its license to manufacture talcum powder due to looming safety concerns.

While the company took the strategic decision to halt the production of certain products in North American regions like the US and Canada in 2020, their presence in the Indian market remains pronounced.
This geographical inconsistency in product availability raises critical questions about global corporate responsibility and ethical business practices.
The proactive stance of Maharashtra's FDA, though laudable, bears the brunt of criticism for its timing. A two-year lapse in identifying and addressing potential hazards, epecially in products catered to vulnerable infants, underscores the dire need for more agile and responsive regulatory mechanisms.

The ongoing saga of Johnson & Johnson serves as a potent testament to the complex interplay between corporate pursuits and ethical imperatives.
As we navigate an era defined by information and empowerment, it becomes imperative for consumers to stay informed, vigilant, and ready to hold powerful entities accountable.
For businesses, especially those in the realm of public health, this episode reinforces the timeless virtues of transparency, ethical diligence, and an unwavering commitment to consumer safety.
Source: 1. The New York Times;
Source: 2: BBC News

Human Papillomavirus (HPV) stands as one of the most widespread and multifaceted groups of viruses affecting millions globally. Its implications...

Congratulations to all the students who have successfully completed their 12th standard! As you embark on the journey of higher studies, it is...

As a student who has completed 12th standard with a physics, chemistry, math or biology (PCMB) stream and is considering higher studies in the field...